What is a clinical trial?
Quotient runs phase I clinical trials, which are the first in a series of four stages of testing new treatments in humans.
These treatments have already been through thorough tests and in some cases, may be new combinations of medicines that are already available. People participate in clinical trials for a variety of reasons. Healthy volunteers say they participate to help others and to contribute to moving science forward. Participants with an illness or disease also participate to help others, but also to possibly receive the newest treatment and to have the additional care and attention from the clinical trial staff. Clinical trials offer hope for many people and an opportunity to help researchers find better treatments for others in the future.
The four phases of clinical testing
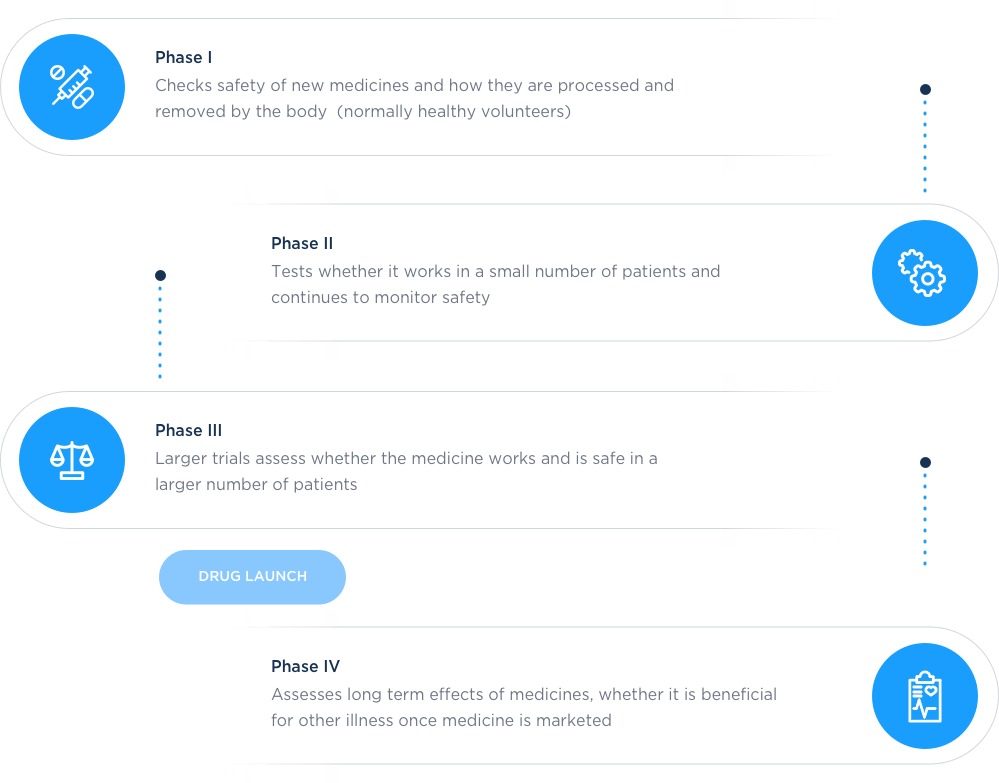
What is a phase I clinical trial?
Phase I clinical trials are designed to understand the safety of the trial test medicine:
-
How much of the trial test medicine can be taken without any undesirable side effects
-
How long it takes for your body to absorb and then get rid of the trial test medicine
-
It may also assess how the trial test medicine interacts with food or other medications
Essentially, we assess the safety of the trial test medicine and how the body processes it.
We are continually monitoring volunteers on our trials. During the trial, once you have taken the test medicine, we may collect blood samples and carry out other tests such as blood pressure and heart rate checks. Your safety and well-being are our primary concern.
Please see below websites for independent bodies that you may find useful: